Thursday, September 28, 2023
Rabies
Wednesday, September 27, 2023
About lumpy skin disease
Lumpy skin disease (LSD) is a devastating disease of cattle and buffalo caused by a capripox virus.
The disease has never been recorded in Australia but is spreading rapidly internationally.
Since 2012, LSD has spread from Africa and the Middle East into south-eastern Europe, affecting European Union (EU) Member countries (Greece and Bulgaria) and several other countries in the Balkans.
LSD was first reported in Asia and the Pacific region in 2019 in north west China, Bangladesh and India. During 2020, LSD continued to spread across continental Asia with many countries including Bhutan, Hong Kong, Myanmar, Nepal, Taiwan, Vietnam and Sri Lanka, reporting outbreaks. In 2021, the disease was confirmed in Vietnam, Thailand, and Malaysia. Indonesia reported confirmed cases in March 2022.
The World Organisation for Animal Health (the OIE) is encouraging members in at-risk areas to initiate vaccination campaigns ahead of virus entry and to continue timely reporting of all outbreaks [1].
The European Union has implemented an intensive (and expensive) vaccination and culling program to halt the spread of the disease.
Victorian Significant Disease Investigation program
With LSD moving through Asia, the risk of this disease entering Australia is increasing. The potential economic impact of an incursion would be considerable due to the disruption of trade in livestock and livestock products, as well as costs associated with disease control and eradication.
With the heightened awareness internationally of LSD, it is important that Australia, with its large dairy and beef export markets, is able to confidently and credibly demonstrate on-going freedom from this disease.
Equally, it is important that veterinary practitioners are aware of the disease and are able to recognise it quickly if an incursion should occur.
To assist with these objectives, the Victorian Significant Disease Investigation (SDI) program supports sample collection and submission from cases where it is appropriate to consider LSD as a potential differential diagnosis.
To be eligible for investigation under the SDI program, cases must be:
- cattle or buffalo of any age
- resident in Victoria
- showing multiple, cutaneous skin lesions.
Samples from single animals are eligible, but cases involving several cattle on the same property are preferred.
Samples to collect:
- Skin lesions (excision or biopsy) – One sample in saline and a duplicate in formalin,
- Blood – One each of clotted/serum (red/gold top) tube and EDTA blood tube.
Submission, documentation and approvals process will be as per usual for a SDI investigation. Always discuss the case with your local department veterinary officer before submission.
Understand the signs of lumpy skin disease
The incubation period for lumpy skin disease is between 4 and 14 days post-infection.
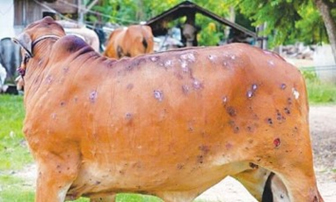
After an initial period of high fever (41°C) and swollen lymph glands, the animal may develop large, firm nodules that are up to 5 cm in diameter in the skin.
These can be found all over the body, but particularly on the:
- head
- neck
- udder
- scrotum
- perineum.
The nodules may become necrotic and ulcerate, leading to an increased risk of flystrike.


Dairy cattle in peak production are often the most severely affected with a marked decrease in milk production. Depression, anorexia, rhinitis, conjunctivitis and excess salivation may also be observed.
In severely affected animals, necrotic lesions can also develop in the respiratory and gastrointestinal tract.
The disease can be subclinical (up to 50% of cases in an outbreak) or may be very severe or even fatal. Morbidity varies between 5 to 45% and mortality rate usually remains below 10% but both rates can be considerably higher when an outbreak occurs in a naïve cattle population.
Other diseases of cattle that could look like lumpy skin disease
- Ringworm and infection with other dermatophytes
- Dermatophilus infection
- Cutaneous leucosis
- Parapox (bovine popular stomatitis)
- Bovine herpes mammilitis
- Pseudo lumpy skin disease (bovine herpesvirus 2)
- Photosensitisation
- Insect bites
- Urticaria
- Demodectic mange
- Trauma, including burns
- Actinobacillosis (cutaneous form due to infection with Actinobacillus lignieresi)
Lumpy skin disease – information for veterinarians
Lumpy skin disease is caused by a Capripoxvirus that affects cattle and buffalo. The disease has never been recorded in Australia, but internationally it is spreading rapidly.
The heightened risk of an incursion in Australia
Although lumpy skin disease is not present in Australia, its recent detection in Indonesia and ongoing spread throughout neighboring Asian countries is very concerning.
Lumpy skin disease would be costly and challenging to control or eradicate should it reach Australia, with wide-ranging and significant long-term impacts for our livestock industries.
Like Japanese encephalitis and other vector-borne diseases, unless an incursion is detected early, its eradication or elimination (eradication in a defined geographical area) would be difficult.
Australia’s current response policy for lumpy skin disease is to eradicate it in the shortest possible time, by the destruction of infected animals and contaminated products, quarantine and movement controls, insect vector control and other activities.
A vaccine is not currently available (or its use permitted) in Australia, although vaccines are in use internationally with varying success in terms of disease control.
The economic impact of an incursion in Australia would be considerable due to the disruption of trade in livestock and livestock products (including products from sheep and goats, not just cattle), as well as costs associated with disease control and eradication.
Continued vigilance is essential in order to detect an incursion as quickly as possible, and thereby protecting the health of the Australian cattle herd, as well as Australia’s export trade in livestock and livestock products.
 Clinical signs
Clinical signs
Clinically, disease may range between subclinical disease to severe illness or death.
Initial clinical signs may include fever (40-41.5 0C), depression, anorexia, and reluctance to move. Rhinitis, conjunctivitis and excess salivation may also be apparent.
Cattle may develop large, firm nodules (up to 5 cm in diameter) in the skin. These can be found over the entire animal, but are found particularly on the head, neck, udder, scrotum and perineum. The nodules may become necrotic and ulcerate, leading to an increased risk of secondary bacterial infections and flystrike. In severe cases, the nodules can fall away leaving full-skin thickness holes in the animals hide.
Affected bulls may not work due to painful lesions on their prepuce, and cows may abort or become anestrus. Live neonates or aborted fetuses from infected cows may also present with skin lesions.
In severely affected animals, lesions can also develop in the respiratory and gastrointestinal tract. Pneumonia is a common, and often fatal, complication.
Bos taurus cattle are generally more susceptible than Bos indicus cattle. Jersey, Guernsey, Friesian and Ayrshire breeds are particularly susceptible.
Morbidity rates vary greatly, ranging between 1-95%. Mortality rates are usually 1-5%, but have been reported as high as 75%.
Disease transmission
Lumpy skin disease virus is spread primarily by biting insects such as flies and mosquitoes, and possibly ticks. It can also be transmitted by fomites, and in some cases, from animal to animal.
The virus is present in high concentrations in the skin nodules and scabs on affected animals, and can be isolated from blood, saliva, ocular and nasal discharges and semen.
Virus can be found in blood for up to 21 days post-infection. Shedding in semen may continue for at least 42 days post-infection.
Lumpy Skin Disease is not Zoonotic
The virus is highly host specific and does not cause disease in humans.
There is no risk from consuming beef or dairy products.
Differential diagnoses
Differential diagnoses include:
- urticaria
- pseudo lumpy skin disease (bovineherpes mammalitis; herpesvirus 2)
- bovine papular stomatitis (parapoxvirus)
- dermatophytosis (Trichophyton spp., Microsporum spp.)
- pseudo cowpox (parapoxvirus)
- vaccinia virus and cowpox virus (orthopoxviruses)
- streptothricosis (Dermatophilus congolensis)
- demodicosis (Demodex bovis)
- insect or tick bites
- onchocercosis (Onchocerca spp.)
- besnoitiosis (Besnotia besnoiti)
- cutaneous actinobacillosis (Actinobacillus lignieresi)
- rinderpest
- Hypoderma bovis infection
- photosensitization
- skin tuberculosis.
Suspicion of Lumpy Skin Disease
Lumpy skin disease is a notifiable disease. All suspected cases must be reported to Agriculture Victoria on the Emergency Animal Disease Hotline on 1800 675 888 or to local Agriculture Victoria Animal Health and Welfare staff immediately.
Sample collection
Diagnosis of lumpy skin disease is based primarily on detection of the virus in lesions. Detection of antibody in serum may also aid diagnosis.
Specimens that should be collected from live animals include:
- blood (from animals with fever)
- serum
- nodular fluid
- skin biopsies of suggestive lesions
- scabs, and
- skin scrapings from lesions.
At necropsy, a range of samples, both fresh and fixed, should be taken from skin lesions, lesions in the respiratory and gastrointestinal tracts, and other internal organs.
Following the initial diagnosis of lumpy skin disease, a more restricted sample set, still based on sampling lesions, may be defined
Tuesday, September 26, 2023
Anthrax in animals
Actions to take if you suspect anthrax
It is essential that, if livestock die suddenly and without an obvious cause:
- Report the incident immediately to your private vet or Agriculture Victoria Animal Health and Welfare (AHW) staff.
- Don't move the carcass.
- Get the carcass tested for anthrax by your private vet or our AHW staff.
If you suspect an animal may have died from anthrax, immediately contact your private vet or local AHW staff.
- You can also call the Customer Call Centre on 136 186.
- If you cannot speak directly with an officer from Animal Health and Welfare do not leave a message. Instead, immediately ring the all-hours Emergency Animal Disease Watch Hotline on 1800 675 888.
What is anthrax?
Anthrax is an infectious bacterial disease of animals, caused by the spore-forming bacteria Bacillus anthracis. It can affect humans and a wide range of animals. Nearly all cases in Victoria have been seen in livestock, particularly cattle and sheep.
Signs of anthrax in livestock
Cattle and sheep with anthrax generally die suddenly.
Just before death, animals may show signs of high fever. Blood may be present around the nose, mouth and anus of carcasses. In many cases you may not see this sign, so it should not be relied on to diagnose anthrax. If livestock die suddenly, even when there is no history of anthrax on the property, anthrax could potentially be the cause.
To prevent a large-scale anthrax incident, it is critically important that the carcasses of cattle and sheep that die suddenly without any other obvious cause are tested for anthrax before they are moved. This reduces the risk of human exposure and minimises contamination of the affected property if anthrax is confirmed.
Where anthrax occurs
Anthrax is well known to occur intermittently in grazing livestock and there have been sporadic cases in Victoria, New South Wales and Queensland in recent decades.
For Victoria, most of the recent cases have been in northern parts of the state and have largely involved cattle. In the past, anthrax has also occurred in Gippsland and South-West Victoria.
For more information please visit the Anthrax — Biosecurity advisory page.
Time of year anthrax occurs
Anthrax usually appears during the warmer months. However, cases of anthrax have occurred, and may occur, at any time of year in Victoria.
Testing for anthrax
Appropriate samples can be collected and tested on farm using the hand-held immunochromatographic test (ICT) with results available within 15 minutes. Further confirmatory testing is usually undertaken at a laboratory, taking approximately 24 hours. This testing will be carried out at no cost to the farmer.
To encourage this, cattle, sheep and goat producers are eligible for a one-off industry-funded incentive payment of $1,000 (cattle) or $500 (sheep or goat) following a positive diagnosis of anthrax if the:
- cattle, sheep or goat carcass have not been moved from the death site, and
- producer reports the case to AgVic or their private veterinarian for testing, and
- animal is found to be the first anthrax case associated with an outbreak (only one payment for an outbreak where multiple farms are affected).
- Note: if more than one species from one producer has a positive diagnosis, only one incentive payment will be made.
If the case occurs on a dairy farm, the dairy processor is advised. Relevant food safety and public health agencies are also routinely notified.
Movement of animals and animal products from the farm is suspended while anthrax testing is carried out.
Livestock producers may be eligible for disease investigation subsidies under the Significant Disease Investigation Program, in the event the death is not associated with anthrax.
Funding of testing for anthrax by private vets
To encourage anthrax exclusion testing, particularly in areas of Victoria with a history of anthrax, we will pay private vets to carry out anthrax testing.
Where a case of anthrax is confirmed after veterinary examination of affected animals and laboratory testing of samples — the affected property is:
- quarantined
- potentially exposed stock are vaccinated
- dead animals are safely disposed of (usually by burning)
- contaminated sites disinfected.
The quarantine is not released until at least 20 days have elapsed since the last anthrax case and at least 20 days have passed since the last round of vaccinations on the property (whichever is later).
Most incidents of anthrax involve single isolated cases, and 'quarantine, vaccination, disposal and decontamination measures prevent further cases. Occasionally, larger-scale outbreaks occur in Victoria, such as those in 1997 and 2007. Vaccination across a wider area is usually required to control larger outbreaks.
Preventing anthrax
If you are in an anthrax-prone area you should contact us if you wish to undertake voluntary preventive vaccination against anthrax.
Risks to people from anthrax in animals
Very few human cases of anthrax have been reported in Victoria.
The greatest risk is to those who handle dead livestock such as farmers, vets and knackery workers.
The last documented case of human anthrax in Victoria was in 2007 when a knackery worker, who had contact with an infected carcass, developed the skin form of anthrax. He was treated with antibiotics and recovered.
Measures taken by us to prepare for an outbreak
The measures taken in Victoria to deal with occurrences of anthrax in farm animals are designed to:
- limit disease outbreaks
- protect domestic and export markets for livestock and their products
- safeguard public health.
We have an all-hours Emergency Animal Disease Watch Hotline for the immediate reporting by farmers and veterinarians of suspected cases of anthrax.
We have comprehensive policy and operational procedures for the management of anthrax, which cover:
- notification
- quarantine
- disinfection
- disposal of carcasses and animal products
- surveillance
- vaccination.
We keep a stock of anthrax vaccine for use in an outbreak. Vaccination carried out in response to a case of anthrax in the area is carried out free of charge.
Australian veterinary authorities maintain emergency plans, based on the Australian Veterinary Emergency Plan (AUSVETPLAN), for the control of a large-scale anthrax outbreak. These plans include collaborative arrangements with a range of government authorities and industry organisations.
Monday, September 25, 2023
What is Rabies?
Rabies is a preventable viral disease most often transmitted through the bite of a rabid animal. The rabies virus infects the central nervous system of mammals, ultimately causing disease in the brain and death. The vast majority of rabies cases reported to the Centers for Disease Control and Prevention (CDC) each year occur in wild animals like bats, raccoons, skunks, and foxes, although any mammal can get rabies.
The Rabies Virus
Rabies virus belongs to the order Mononegavirales, viruses with a nonsegmented, negative-stranded RNA genomes. Within this group, viruses with a distinct “bullet” shape are classified in the Rhabdoviridae family, which includes at least three genera of animal viruses, Lyssavirus, Ephemerovirus, and Vesiculovirus. The genus Lyssavirus includes rabies virus, Lagos bat, Mokola virus, Duvenhage virus, European bat virus 1 & 2 and Australian bat virus.
Structure
Rhabdoviruses are approximately 180 nm long and 75 nm wide. The rabies genome encodes five proteins: nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G) and polymerase (L). All rhabdoviruses have two major structural components: a helical ribonucleoprotein core (RNP) and a surrounding envelope. In the RNP, genomic RNA is tightly encased by the nucleoprotein. Two other viral proteins, the phospoprotein and the large protein (L-protein or polymerase) are associated with the RNP.
The glycoprotein forms approximately 400 trimeric spikes which are tightly arranged on the surface of the virus. The M protein is associated both with the envelope and the RNP and may be the central protein of rhabdovirus assembly. The basic structure and composition of rabies virus is depicted in the longitudinal diagram below.
Rabies is an RNA virus. The genome encodes 5 proteins designated as N, P, M, G, and L. The order and relative size of the genes in the genome are shown in the figure below. The arrangement of these proteins and the RNA genome determine the structure of the rabies virus.
Replication
The fusion of the rabies virus envelope to the host cell membrane (adsorption) initiates the infection process. The interaction of the G protein and specific cell surface receptors may be involved.
After adsorption, the virus penetrates the host cell and enters the cytoplasm. The virions aggregate in the large endosomes (cytoplasmic vesicles). The viral membranes fuse to the endosomal membranes, causing the release of viral RNP into the cytoplasm (uncoating). Because lyssaviruses have a linear single-negative-stranded ribonucleic acid (RNA) genome, messenger RNAs (mRNAs) must be transcribed to permit virus replication.
A viral-encoded polymerase (L gene) transcribes the genomic strand of rabies RNA into leader RNA and five capped and polyadenylated mRNAs, which are translated into proteins. Translation, which involves the synthesis of the N, P, M, G and L proteins, occurs on free ribosomes in the cytoplasm. Although G protein synthesis is initiated on free ribosomes, completion of synthesis and glycosylation (processing of the glycoprotein), occurs in the endoplamsic reticulum (ER) and Golgi apparatus. The intracellular ratio of leader RNA to N protein regulates the switch from transcription to replication. When this switch is activated, replication of the viral genome begins. The first step in viral replication is synthesis of full-length copies (postive strands) of the viral genome. When the switch to replication occurs, RNA transcription becomes “non-stop” and stop codons are ignored. The viral polymerase enters a single site on the 3’ end of the genome, and proceeds to synthesize full-length copies of the genome. These positive strands of rabies RNA serve as templates for synthesis of full-length negative strands of the viral genome.
During the assembly process, the N-P-L complex encapsulates negative-stranded genomic RNA to form the RNP core, and the M protein forms a capsule, or matrix, around the RNP. The RNP-M complex migrates to an area of the plasma membrane containing glycoprotein inserts, and the M-protein initiates coiling. The M-RNP complex binds with the glycoprotein, and the completed virus buds from the plasma membrane. Within the central nervous system (CNS), there is preferential viral budding from plasma membranes. Conversely, virus in the salivary glands buds primarily from the cell membrane into the acinar lumen. Viral budding into the salivary gland and virus-induced aggressive biting-behavior in the host animal maximize chances of viral infection of a new host.
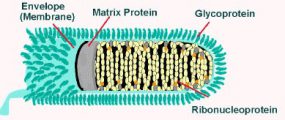
Rabies virions are bullet-shaped with 10-nm spike-like glycoprotein peplomers covering the surface. The ribonucleoprotein is composed of RNA encased in nucleoprotein -(), phosphorylated or phosphoprotein -Illistration of virus, and polymerase -virus.
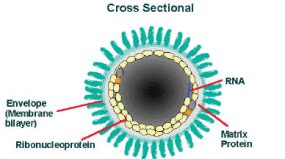
The cross-sectional diagram demonstrates the concentric layers: envelope membrane bilayer, M protein, and tightly coiled encased genomic RNA.

The rabies virus genome is single-stranded, antisense, nonsegmented, RNA of approximately 12 kb. There is a leader-sequence (LDR) of approximately 50 nucleotides, followed by N, P, M, G, and L genes.
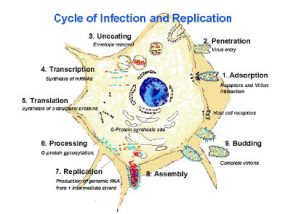
1: Adsorption (receptors and virion interation). 2: Penetration (virus entry). 3: Uncoating (envelope removal). 4. Transcription (synthesis of mRNAs). 5. Translation (Synthesis of structural proteins). 6. Processing (G-protein gycosylation). 7. Replication (production of genomic RNA from intermediate strand. 8. Assembly. 9: Budding (complete virions).
Saturday, September 23, 2023
Pregnancy testing of beef cattle
Pregnancy testing is one method of monitoring reproductive efficiency and detecting any problems early in the breeding cycle.
The key to profitability for all beef breeding enterprises is high reproductive efficiency. This means achieving:
- 95% calves weaned to cows joined
- an average calving interval of 12 months
- a calving spread of 10 weeks or less
These are all realistic objectives in Victoria. This can be achieved by early detection of pregnant cows.
Pregnancy testing for cows
Manual rectal palpation is a proven effective and reliable technique. Using this method pregnancies can be reliably detected as early as 6 weeks.
Ultrasound has also become commonly used. The main advantage of ultrasound pregnancy diagnosis is it reduces operator fatigue and injuries. It is important to recognise that females identified as non-pregnant by ultrasound must then be confirmed by rectal palpation. As ultrasound is not as specific for non-pregnant’ s. Both methods can be used to age foetus’s however accuracy of ageing reduces as the foetus age increases.
Experienced operators can age foetuses accurately particularly in the first 4 months of pregnancy. Pregnancy testing is normally carried out 8-10 weeks after the end of mating. Cows need to be restrained in a race — it may not be necessary to headbail each one. In well designed yards and with labour provided to keep cattle moving into the race, up to 60 cows can be pregnancy-tested per hour.
Benefits of pregnancy testing your cows
Early detection of non-pregnant cows is the main benefit from pregnancy testing, but there are others.
Estimating calving dates
In many cases, the age of the calf and the likely calving date can be estimated during rectal palpation. Cows expected to calve early can then be separated from cows expected to calve late. This can provide a useful basis on which to cull cows if it is necessary to reduce herd size, perhaps in times of feed shortage. The calving spread can also be quickly reduced if late-calving cows are replaced with heifers that conceived early.
Detecting infertility and other abnormalities in cows
Various abnormalities responsible for infertility in cows can also be identified. The more common of these include cystic ovaries and uterine infection. The occasional freemartin heifer and other abnormalities of the reproductive organs may also be detected during rectal palpation.
Diseases and management problems affecting the whole herd can also be identified much earlier if cattle are pregnancy tested.
Low pregnancy rates in one particular mob, for example, might indicate problems with an individual bull. Poor fertility throughout the whole herd might be caused by an infectious disease, or perhaps inadequate nutrition prior to mating.
After pregnancy testing
Pregnancy testing is of little use as an aid to management unless the information gained is used to make management decisions.
Non-pregnant cows
Feed availability, current beef prices, and the management system used on the property must all be considered when deciding the fate of non-pregnant cattle.
By pregnancy testing, you are in a position to make the best possible decision.
In most situations non-pregnant cows are best culled as soon as possible. The cost of owning and maintaining a beef cow for a year is very high, so it is important that every cow on the property is fully productive. Even if they have calves at foot, non-pregnant cows are only partially productive. Mature cows sometimes fail to conceive after a late calving. Such cows wean the youngest, smallest calves, and are therefore best culled.
Under certain circumstances it might be better not to cull non-pregnant cows immediately. If feed is plentiful, and particularly if beef prices are depressed, the best strategy might be to draft off non-pregnant cows and fatten them for sale at a later date. Alternatively, the most profitable decision might be to sell them after their calves are weaned. It is a common practice to 'bang' the tails (that is, trim about 100mm of hair from the switch) of cows to be culled at some time in the future, so that they can be identified for up to six months. However, it is best to also record ear tag numbers to ensure non-pregnant cows are not lost in herd.
The other option is to re-mate non-pregnant cows, particularly young cows, for a later calving. This strategy may have application on properties that have more than one calving period each year. However, it may result in reduced genetic selection for fertility in the herd. On properties with one calving period, small groups of late calvers create management difficulties.
Where non-pregnant cows are re-mated, good records are needed to ensure that barren cows are not being kept and moved between mobs calving at different times.
On some properties, cows are not pregnancy tested but are simply culled when they reach some predetermined age. Quite often cows culled for age at 9, 10 or 11 are still rearing good calves each year. On the other hand, some of the younger cows retained in the herd fail to conceive.
Old, pregnant cows are obviously more productive than young 'empty' ones. Culling cows on the basis of a pregnancy test is much more efficient than simply culling on age.
Pregnancy diagnosis also has implications for marketing. Cows sold by live weight will attract a higher price if they have been certified by a vet to be non-pregnant. In addition, females sold for future breeding will be worth more if they have had their pregnancy status confirmed. PREGCHECK™ accredited veterinarians can also provide a tail tag indicating the pregnancy status which gives buyers greater confidence.
Non-pregnant heifers
The two main considerations of whether non-pregnant heifers are given a second chance to conceive are the breeding value of the heifers and the cost of carrying the heifers over. When a group of heifers have been reared and mated under similar conditions, those that fail to conceive are less fertile than the group. It is possible that these heifers will fail to conceive if kept for a second joining, or if the heifers conceive, the tendency displayed toward lower fertility may be passed on to heifer daughters.
For maximum profitability, heifers should usually be brought into production as early as possible. The cost of feeding and maintaining non-pregnant heifers for an extra year cannot be justified.. Heifers that fail to conceive at first mating should therefore be culled.
It is desirable to mate heifers for a short period, (6-8 weeks) to ensure a compact calving. It is also a sound practice to mate more heifers than are needed for replacements. Heifers can then be pregnancy tested eight weeks after the end of mating, and only those pregnant need to be kept
Friday, September 22, 2023
Bovine ephemeral fever
Bovine ephemeral fever
Bovine ephemeral fever (BEF) is a disease that affects cattle and occasionally buffaloes and is marked by a short fever, shivering, lameness and muscular stiffness. Also commonly known as 3 day sickness, BEF is an arthropod-borne virus (most likely mosquitoes) and widespread in Queensland.
The disease may cause serious economic losses through deaths, decline in condition, decreased milk production, lowered fertility in bulls, occasional abortions and delays in marketing.
Cause
An arthropod–borne rhabdovirus known as ‘ephemeral fever virus’ or ‘bovine ephemeral fever virus'.
Other names
- 3 day sickness
- BEF
Distribution
- occurs most years in northern Australia.
- usually spreads from north to south.
- governed by season and rainfall; yearly insect distribution
- can occur in herds just south of the usual distribution.
- herd immunity depends on
- location relative to normal distribution
- older animals with previous exposure will be immune
- recent seasonal conditions
- calves have a relative immunity up to approximately 6 months of age.
- location relative to normal distribution
Hosts
Unconfirmed. Thought to be mosquitoes and biting midges (sandflies).
Affected animals
- cattle
- buffaloes
Clinical signs
There are typically 3 recognised stages of bovine ephemeral virus.
The acute febrile stage appears suddenly and is especially noticeable in dairy cattle. Affected cattle are likely to:
- show signs associated with a fever
- have a rectal temperature over 40°C
- shiver (in approximately 50% of cases)
- stand with their backs arched and heads held low, muzzles extended, drooling saliva
- have discharge from eyes and nostrils
- stop feeding and cud chewing
- have reduced milk production, especially in dairy cows.
The second stage is muscular stiffness and lameness in 1 or more limbs. Some secondary bloat may occur due to general inflammation of the abdominal cavity and ruminal stasis. Lameness may shift between limbs and joints may be visibly swollen.
During recovery, most affected animals resume eating and drinking. Animals may go down, with heavy animals in good condition being most affected. Some animals remain down due to muscle damage or damage to the spinal cord from constant struggling.
Post-mortem examination
Post-mortem examination is important to rule out other acute febrile diseases that often occur under the same conditions, such as tick fever. Post-mortem examination can either show small amounts of fibrin-rich fluid, or occasionally, an excess of pinkish to blood stained fluid in the heart sac, chest and abdominal cavities, and joint capsules. Lesions in the lungs may be present in severe cases.
Impacts
Economic
Significant losses can occur because BEF is most severe in more valuable classes of cattle, including:
- bulls
- pregnant and lactating cows
- fat, well-conditioned cattle.
Symptoms usually last only a few days, but the disease can significantly affect the herd's production in the following ways:
- dramatic drop in milk production - over 70% is not uncommon
- milk yield after recovery is often reduced by 15% or more
- lactating cows can dry up completely
- abortion can occur in heavily pregnant cows
- occasional deaths (3%) or prolonged recumbency leading to 'downer syndrome' can occur
- temporary or permanent paralysis may occur as a result of damage to the spinal cord
- most of the herd can be affected
- bulls may be temporarily infertile (up to 8 weeks).
The cost of treatment for affected animals can also be considerable.
How it is spread
While the vectors have not been confirmed, mosquitoes and biting midges (sandflies) are thought to be responsible.
The disease can also be spread by intravenous inoculation of small amounts of blood. BEF is not transmitted by close contact, bodily secretions, or aerosol droplets, nor does it seem to be transmitted in semen. Carriers are not known to occur and the virus is rapidly inactivated in meat.
The spread of the disease depends on the season and weather conditions - rain and prevailing easterly and southerly winds are necessary for the survival and dispersal of vectors. The National Arbovirus Monitoring Program (NAMP) monitors the spread of ephemeral fever virus within Australia.
Risk period
In most years, BEF cases start at the beginning of the wet season in northern Australia, and then spread south and east down the east coast. It then spreads into southern Queensland, and central and coastal New South Wales.
It is less common in drought years, but can occur following an extended period of drought.
Monitoring and action
BEF epidemics are diagnosed on the presence of lameness, muscular stiffness, pain, short fever and rapid spread of the disease through herds.
The virus can often be cultured from a blood sample taken from animals in the early stages of the disease. A PCR test can also identify the presence of the virus, and is most successful when samples are collected in the first few days of clinical disease. Alternatively, 2 blood samples—the first taken during the fever stage and the second 14 days later—can be collected to detect the presence of antibodies to the virus.
Control
Vaccination
There is a modified live vaccine for BEF that provides long-lasting protection. A 2-part vaccine - freeze-dried vaccine with chilled liquid adjuvant - that must be mixed prior to administering, it provides good levels of protection against BEF. While some cattle might still develop mild disease after vaccination, the severity and duration of illness tends to be much lower than in unvaccinated cattle.
The initial vaccine should be administered twice, 2–4 weeks apart, under the skin of the neck for long-lasting protection. To ensure that animals are fully immune before insect populations have the opportunity to breed, you should vaccinate before spring, especially in northern Australia.
An annual booster should be given 8–10 weeks before the BEF season.
Contact your veterinarian for advice on a vaccination program and use of the vaccine.
Treatment
- Anti-inflammatory drugs have been shown to reduce the course of the disease.
- Consult your veterinarian for an appropriate anti-inflammatory drug, considering the withholding period of the drug for meat and milk.
- Most animals will recover if given water, shade and food.
Common Cat disease
Heartworm
Spread by infected mosquitoes, heartworm is increasingly being recognized as an underlying cause of health problems in domestic cats. Cats are an atypical host for heartworms. Despite its name, heartworm primarily causes lung disease in cats. It is an important concern for any cat owner living in areas densely populated by mosquitoes, and prevention should be discussed with a veterinarian.
You may have thought heartworm disease only affects dogs, and it’s true that the infection is less common in cats. The cat is not a natural host for the heartworm parasite, Dirofilaria immitis, and so the heartworm is not likely to complete its entire life cycle. That means that fewer and smaller worms survive, and many do not reach a cat’s heart. The worms that do survive—and the resulting immune reaction that the cat’s body sets up to kill the developing worms—can cause severe health problems.

Causes and Signs of Heartworm Disease
When a mosquito carrying the heartworm parasite, Dirofilaria immitis, bites a cat, larvae are transmitted into the bloodstream. The larvae migrate toward the heart over a period of around four to six months, maturing as they go, then settle in the heart, pulmonary arteries and blood vessels of the lungs. Because a domestic cat is not a natural host for the heartworm parasite, many of the worms die. These—along with the living worms—cause severe inflammatory and immune responses in an infected cat.
Cats of all ages, living in any region, can contract heartworm, but the disease is more prevalent in felines who live in areas densely populated by mosquitoes. Outdoor cats are at greater risk because of increased exposure to mosquitoes. However, indoor cats are also susceptible to mosquito bites, so it’s smart to discuss prevention with your vet. The heartworm infection can be especially life-threatening to kittens and older cats.
The following signs may indicate that your cat has been infected:
- Persistent cough
- Breathing difficulties (panting, wheezing, rapid or open-mouthed breathing)
- Depression
- Loss of appetite
- Weight loss
- Sporadic vomiting
- Lethargy
- Sudden death
Breathing difficulties that occur in the first stage of heartworm disease, caused by worms newly arriving in the heart and lungs, were likely previously diagnosed as feline asthma or bronchitis. However, these breathing problems are now thought to have actually been due to what is now called heartworm-associated respiratory disease (HARD).
Heartworm Prevention
- There are several FDA-approved medications available that reliably prevent feline heartworm infection. Check with your vet and please remember, it’s recommended that cats are screened for heartworm infection with blood tests before being given any type of preventative medication.
- It’s also a good idea to limit your cat’s exposure to mosquito-infested areas and bring her in for preventative screenings during vet visits.
- Regular checkups are key to detecting early infections and can give your cat a good chance at recovery.
Diagnosing Heartworm Disease
Heartworm disease is not as easily diagnosed in cats as it is in dogs.
- Routine testing requires a combination of blood tests.
- When cats show signs of respiratory difficulty and heartworm is suspected, diagnosis is usually based on a cat’s history, physical examination, radiographs, echocardiogram and blood tests.
Treating Heartworm Disease
There are currently no products in the United States approved for treating feline heartworm infection. The good news is that many heartworm-infected cats are able to fight the infection themselves, and can be monitored with radiographs every few months, while waiting out the worms’ lifespan. If an infected cat shows symptoms of lung disease, the cat can be given a cortisone-like medication as needed. Medication can also be given to help control coughing and vomiting.
Although some cats are able to fight the infection on their own, the following can occur if heartworms are not monitored and treated:
- Damage to walls of heart
- Damage to pulmonary blood vessels
- Possible obstruction of blood flow through pulmonary arteries
- Impaired breathing
- Heart and lung failure
- Kidney and liver damage
- Sudden death
-
Lumpy cow skin disease is a concern for cattle owners, leading to significant economic losses worldwide. Thus, many are eager to know wh...



